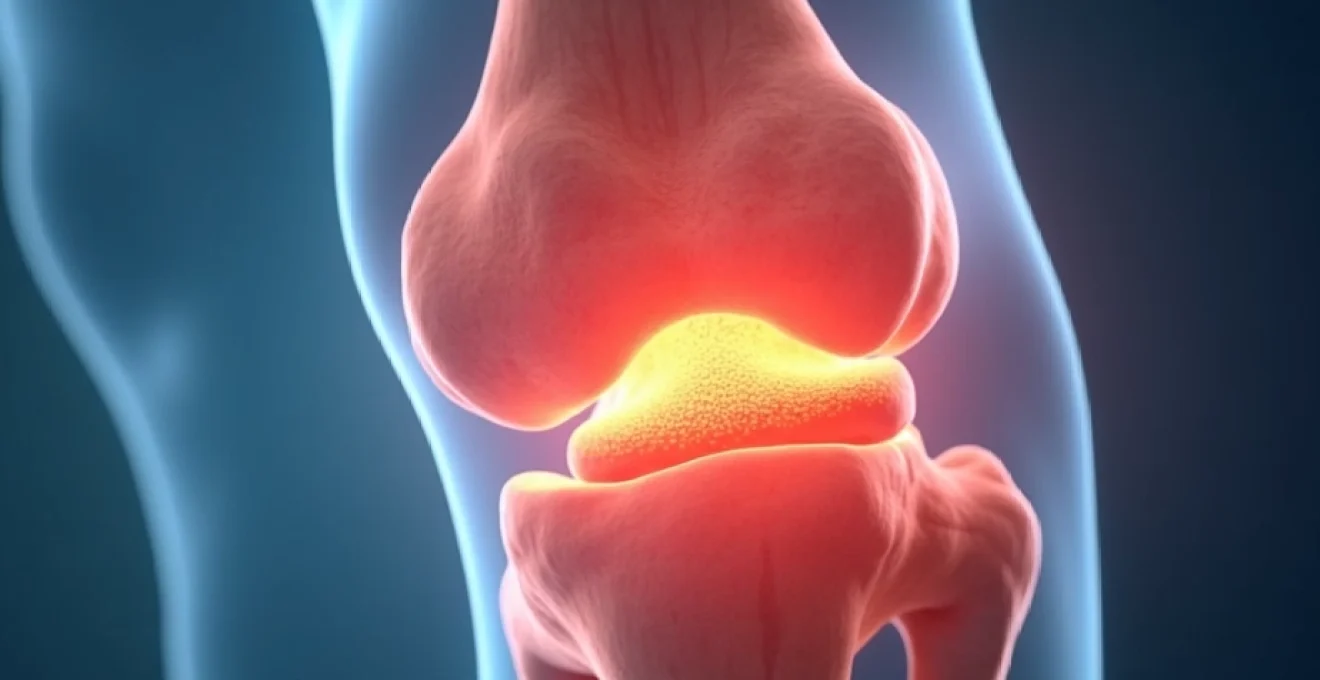
Tibial indentations represent a complex clinical manifestation that can signal various underlying pathological processes affecting the shin bone’s structural integrity. These depressions or concavities in the tibial surface may arise from traumatic injuries, metabolic disorders, or systemic conditions that compromise bone density and architecture. Understanding the multifaceted nature of tibial indentations requires a comprehensive examination of bone biomechanics, pathophysiology, and diagnostic approaches. Healthcare professionals must possess detailed knowledge of the tibia’s anatomical characteristics and the mechanisms that lead to bone deformation to provide accurate assessments and appropriate treatment strategies. The clinical significance of shin bone indentations extends beyond mere cosmetic concerns, as they often indicate deeper structural abnormalities that require thorough investigation and intervention.
Anatomical structure and biomechanics of tibial bone architecture
The tibia, commonly known as the shin bone, represents the larger and stronger of the two lower leg bones, bearing the primary weight-bearing load of the human body. This robust bone exhibits a complex architectural design that optimises both strength and flexibility under various mechanical stresses. The tibial structure incorporates sophisticated engineering principles that allow it to withstand forces up to five times body weight during activities such as running or jumping whilst maintaining structural integrity throughout decades of use.
Cortical and cancellous bone distribution in the tibia
The tibial bone architecture demonstrates a carefully orchestrated distribution of cortical and cancellous bone tissue that maximises structural efficiency. Cortical bone, comprising approximately 80% of the total bone mass, forms the dense outer shell that provides primary mechanical support and resistance to bending forces. This compact bone tissue exhibits a porosity of only 5-10%, creating an exceptionally strong framework that can withstand compressive loads exceeding 200 MPa. The cortical thickness varies significantly along the tibial shaft, with the mid-shaft region displaying the greatest cortical density to accommodate peak stress concentrations during locomotion.
Cancellous bone, also termed trabecular bone, occupies the interior spaces and demonstrates a sponge-like architecture with 50-90% porosity. This metabolically active tissue serves as the body’s calcium reservoir whilst providing shock absorption capabilities that complement the rigid cortical shell. The trabecular network aligns along primary stress trajectories, creating a sophisticated internal scaffolding system that distributes forces efficiently throughout the bone structure.
Periosteal and endosteal surface characteristics
The periosteum, a fibrous membrane enveloping the tibial surface, plays a crucial role in bone health and repair mechanisms. This highly innervated and vascularised tissue contains osteoblast precursor cells that facilitate bone growth, remodelling, and healing processes. The periosteal surface area in the tibia exceeds 150 square centimetres in adults, providing extensive coverage for metabolic activities and structural maintenance. Disruption of periosteal integrity can significantly impact the bone’s ability to respond to mechanical stresses and repair microtrauma.
The endosteal surface, lining the internal medullary cavity, maintains close communication with the bone marrow and serves as another source of osteogenic cells. This interface facilitates the exchange of nutrients, hormones, and cellular signals that regulate bone metabolism and homeostasis throughout life.
Load-bearing mechanics and stress distribution patterns
Tibial load-bearing mechanics involve complex interactions between compressive, tensile, and shear forces that vary according to activity level and body position. During normal walking, the tibia experiences peak compressive forces of approximately 2.5 times body weight , whilst high-impact activities can generate forces exceeding 8-12 times body weight. These mechanical loads create specific stress distribution patterns that influence bone remodelling and adaptation over time.
The bone’s ability to adapt to mechanical demands through Wolff’s Law ensures optimal tissue distribution where stresses are highest. Areas subjected to repetitive loading develop increased bone density and cortical thickness, whilst regions experiencing reduced mechanical stimulation may undergo resorption and weakening. This adaptive process requires 3-4 months to complete a full remodelling cycle, during which temporary weakness may occur if loading patterns change dramatically.
Tibial shaft geometry and Cross-Sectional variations
The tibial shaft exhibits distinct geometric variations along its length that reflect functional adaptations to mechanical demands. The proximal region displays a triangular cross-section with prominent medial and lateral borders, whilst the middle third assumes a more circular configuration. This geometric evolution optimises the bone’s resistance to bending and torsional forces whilst minimising material usage. The cross-sectional moment of inertia, a key measure of bending resistance, reaches maximum values at the mid-shaft level where mechanical stresses typically peak during locomotion.
Pathophysiology of tibial indentation formation
Tibial indentation formation involves complex cellular and molecular processes that disrupt normal bone architecture and compromise structural integrity. These pathological changes may occur through various mechanisms, including accelerated bone resorption, impaired bone formation, or direct mechanical disruption of the cortical shell. Understanding these underlying processes enables clinicians to identify potential causes and develop targeted treatment strategies that address the root pathophysiology rather than merely treating superficial symptoms.
Osteoclastic activity and bone resorption mechanisms
Excessive osteoclastic activity represents a primary mechanism underlying tibial indentation formation, particularly in metabolic bone diseases. Osteoclasts, multinucleated cells responsible for bone resorption, can become hyperactivated due to hormonal imbalances, inflammatory cytokines, or genetic factors. These cells create acidic microenvironments with pH levels below 4.5, dissolving hydroxyapatite crystals and collagen matrix components. When osteoclastic activity exceeds osteoblastic bone formation, localised areas of bone loss develop, potentially creating visible indentations in the tibial cortex.
The resorption process involves sophisticated molecular signalling pathways, including the RANK-RANKL-OPG system that regulates osteoclast differentiation and activation. Dysregulation of these pathways can lead to pathological bone loss rates exceeding 3-5% annually , compared to normal physiological turnover rates of 2-3% per year.
Haversian system disruption and osteon remodelling
The Haversian system, comprising osteons as the fundamental structural units of cortical bone, may undergo pathological remodelling that contributes to surface irregularities. Each osteon contains a central canal surrounded by concentric lamellae of mineralised collagen, creating a cylindrical structure approximately 200-250 micrometers in diameter. Disruption of normal osteon architecture through accelerated remodelling or incomplete mineralisation can create focal weak points that may manifest as surface depressions.
Abnormal osteon remodelling often occurs in response to mechanical stress concentrations, microtrauma, or systemic factors that alter bone metabolism. The remodelling process typically requires 3-6 months for completion, during which the affected bone region experiences temporary weakness and increased susceptibility to deformation.
Vascular compromise and bone necrosis pathways
Vascular compromise represents another significant pathway leading to tibial indentation formation, particularly following traumatic injuries or conditions that impair bone blood supply. The tibial blood supply depends on nutrient arteries, periosteal vessels, and metaphyseal circulation that collectively deliver oxygen and nutrients essential for bone cell survival. Disruption of this vascular network can lead to localised bone necrosis and subsequent collapse of the cortical structure.
Bone tissue demonstrates relatively high metabolic demands, consuming approximately 10-15% of cardiac output despite representing only 15% of total body weight. Vascular compromise lasting more than 6-8 hours can result in irreversible bone cell death and structural deterioration that may manifest as surface irregularities or indentations.
Inflammatory cascade effects on tibial cortex
Inflammatory processes can significantly impact tibial cortical integrity through various cellular and molecular mechanisms. Pro-inflammatory cytokines such as interleukin-1, tumor necrosis factor-alpha, and interleukin-6 can directly stimulate osteoclast activity whilst inhibiting osteoblast function. This inflammatory imbalance promotes net bone loss and structural weakening that may contribute to surface deformation. Chronic inflammatory conditions affecting the lower extremity can create persistent cytokine exposure that gradually undermines bone quality and predisposes to indentation formation.
Traumatic aetiologies of shin bone depression
Traumatic injuries represent one of the most common causes of tibial indentations, encompassing a broad spectrum of mechanisms from acute high-energy impacts to repetitive microtrauma over extended periods. Direct trauma to the shin bone can create immediate structural damage that manifests as visible depressions, whilst indirect forces may cause stress concentrations that gradually weaken specific areas of the cortical bone. Understanding the relationship between trauma mechanisms and resulting bone deformation patterns enables clinicians to anticipate associated injuries and develop comprehensive treatment approaches.
High-energy trauma, such as motor vehicle accidents or falls from significant heights, can generate compressive forces exceeding the bone’s ultimate strength of approximately 170-190 MPa. These forces can create comminuted fractures, cortical impaction, or direct crushing injuries that result in permanent bone deformation. The tibia’s subcutaneous location along the medial border makes it particularly vulnerable to direct impact injuries, as minimal soft tissue coverage provides limited protection against external forces.
Repetitive microtrauma represents another significant mechanism underlying shin bone depression, particularly in athletes or individuals engaged in high-impact activities. Each loading cycle may cause microscopic damage that accumulates over time when repair processes cannot keep pace with ongoing injury. This phenomenon, commonly observed in conditions such as medial tibial stress syndrome, can lead to cortical weakening and eventual surface irregularities. The bone’s adaptive response to repetitive loading typically requires 6-8 weeks to establish protective remodelling, during which period the risk of structural compromise remains elevated.
Penetrating trauma, including gunshot wounds or sharp object injuries, can create focal areas of bone destruction that heal with irregular surface contours. These injuries often involve contamination and infection risks that further complicate the healing process and may result in permanent structural defects. The presence of foreign material or devitalised bone fragments can impede normal repair mechanisms and contribute to chronic inflammatory responses that perpetuate bone loss.
Understanding the biomechanical principles governing bone failure under various loading conditions enables more accurate prediction of injury patterns and optimal treatment selection for traumatic tibial indentations.
Stress fractures represent a specific subset of traumatic aetiology that deserves particular attention due to their insidious onset and potential for progression to complete fractures. These injuries typically develop in response to repetitive loading that exceeds the bone’s adaptive capacity, creating microscopic cracks that gradually propagate through the cortical structure. The healing response may involve callus formation that creates surface irregularities, particularly if loading continues during the repair process.
Metabolic and systemic disorders affecting tibial integrity
Metabolic and systemic disorders can profoundly impact tibial bone integrity through various pathophysiological mechanisms that alter normal bone metabolism, mineral homeostasis, and cellular function. These conditions often present with subtle clinical signs initially, making early detection challenging but crucial for preventing progressive bone deterioration and associated complications. The tibia’s prominent role in weight-bearing makes it particularly susceptible to the effects of systemic bone diseases, which may manifest as generalised weakening or focal areas of structural compromise.
Osteoporosis and bone mineral density reduction
Osteoporosis represents one of the most prevalent metabolic bone diseases affecting tibial integrity, characterised by reduced bone mineral density and deteriorated microarchitecture that increases fracture risk. This condition affects over 200 million people worldwide , with postmenopausal women experiencing the highest prevalence due to oestrogen deficiency. The tibial cortex undergoes significant thinning in osteoporosis, with cortical thickness reductions of 20-40% commonly observed in severe cases. This cortical thinning can create surface irregularities and indentations, particularly in areas subjected to mechanical stress.
The pathophysiology of osteoporosis involves an imbalance between bone resorption and formation, with osteoclastic activity exceeding osteoblastic bone production. Annual bone loss rates in postmenopausal osteoporosis can reach 3-5%, compared to normal physiological turnover rates of approximately 2%. This accelerated bone loss affects both cortical and trabecular compartments, leading to reduced mechanical strength and increased susceptibility to deformation under normal loading conditions.
Paget’s disease and abnormal bone turnover
Paget’s disease of bone represents a chronic disorder characterised by abnormal and excessive bone turnover that can significantly affect tibial structure and appearance. This condition affects approximately 1-2% of adults over age 50 in developed countries, with the tibia being one of the most commonly affected bones. The disease involves three distinct phases: an initial lytic phase with increased bone resorption, a mixed phase with both resorption and formation, and a final sclerotic phase with predominantly bone formation.
The abnormal bone turnover in Paget’s disease produces structurally inferior bone tissue with irregular architecture and compromised mechanical properties. Affected tibiae may exhibit bowing deformities, surface irregularities, and areas of both excessive bone formation and resorption that create characteristic indentations. The newly formed bone demonstrates a disorganised collagen pattern that reduces overall strength and increases susceptibility to fractures and deformation.
Hyperparathyroidism and calcium metabolism disorders
Hyperparathyroidism and related calcium metabolism disorders can significantly impact tibial bone integrity through disruption of normal mineral homeostasis and bone remodelling processes. Primary hyperparathyroidism, affecting approximately 0.1-0.4% of the population , leads to excessive parathyroid hormone production that stimulates osteoclastic bone resorption and reduces bone mineral density. The tibia, as a major weight-bearing bone, demonstrates particular vulnerability to the effects of chronic calcium depletion.
Parathyroid hormone excess creates a state of accelerated bone turnover with net bone loss, particularly affecting cortical bone sites. The tibial cortex may develop areas of subperiosteal resorption, creating surface irregularities and indentations that reflect the underlying metabolic disturbance. Secondary hyperparathyroidism, commonly associated with chronic kidney disease or vitamin D deficiency, can produce similar effects on tibial bone structure.
Malnutrition and vitamin D deficiency impact
Malnutrition and vitamin D deficiency represent significant risk factors for tibial bone deterioration, particularly in vulnerable populations such as the elderly, institutionalised individuals, or those with limited sun exposure. Vitamin D deficiency affects over 1 billion people worldwide , contributing to impaired calcium absorption and secondary hyperparathyroidism that promotes bone loss. The tibial cortex demonstrates particular sensitivity to nutritional deficiencies due to its high metabolic demands and continuous remodelling requirements.
Protein malnutrition can significantly impact bone matrix formation and mineralisation processes, leading to reduced bone quality and increased susceptibility to deformation. The organic matrix comprises approximately 35% of total bone volume, and adequate protein intake is essential for maintaining collagen synthesis and bone cell function. Severe malnutrition can result in osteomalacia, a condition characterised by inadequate bone mineralisation that may manifest as bone deformities and surface irregularities including tibial indentations.
The complex interplay between nutrition, hormonal regulation, and bone metabolism underscores the importance of comprehensive metabolic evaluation in patients presenting with unexplained tibial indentations.
Clinical assessment techniques for tibial indentations
Comprehensive clinical assessment of tibial indentations requires systematic examination techniques that evaluate both local bone pathology and underlying systemic conditions that may contribute to structural abnormalities. The assessment process begins with detailed history-taking to identify potential traumatic events, systemic diseases, medications, and lifestyle factors that could predispose to bone abnormalities. Physical examination must incorporate specific techniques for evaluating bone integrity, soft tissue conditions, and neurovascular status to develop a complete clinical picture.
Visual inspection represents the initial step in clinical assessment, examining the tibial contour for asymmetry, surface irregularities, or obvious deformities. Proper lighting and patient positioning facilitate accurate observation of subtle changes in bone architecture that might otherwise be overlooked. The examiner should assess both legs simultaneously to identify unilateral abnormalities and document the precise location, dimensions, and characteristics of any indentations observed. Photography may prove valuable for documenting baseline appearance and monitoring progression over time.
Palpation techniques provide crucial information about bone texture, temperature,
tenderness, and any associated soft tissue changes that may indicate underlying pathological processes. Systematic palpation should begin proximally and progress distally, comparing findings between both limbs to identify asymmetries or abnormal responses. The examiner should apply graduated pressure to assess bone pain, localised tenderness, or areas of increased warmth that might suggest active bone pathology or inflammatory processes.
Functional assessment techniques evaluate the impact of tibial indentations on weight-bearing capacity, gait mechanics, and overall lower extremity function. Observing the patient during walking, stair climbing, and single-leg stance provides valuable insights into compensatory movement patterns and functional limitations. Range of motion testing of adjacent joints helps identify secondary effects of tibial abnormalities on overall limb function and mobility.
The clinical examination should incorporate specialised provocation tests designed to identify specific pathological conditions that may contribute to tibial indentation formation. Percussion testing along the tibial shaft can reveal areas of increased sensitivity or pain that may indicate stress fractures or other structural abnormalities not immediately apparent through visual inspection. The tuning fork test, applied to bony prominences, can help differentiate between soft tissue and osseous pathology through vibratory sensation assessment.
Advanced imaging modalities and diagnostic protocols
Advanced imaging represents an essential component of comprehensive tibial indentation evaluation, providing detailed visualisation of bone architecture, soft tissue involvement, and underlying pathological processes that may not be apparent through clinical examination alone. The selection of appropriate imaging modalities depends on the suspected aetiology, clinical presentation, and specific diagnostic questions that need to be addressed. Modern imaging techniques offer unprecedented resolution and specificity for identifying subtle bone abnormalities and monitoring treatment responses over time.
High-resolution CT scanning and 3D reconstruction analysis
High-resolution computed tomography scanning provides exceptional detail of cortical bone architecture and enables precise quantification of tibial indentation characteristics. Modern CT scanners achieve spatial resolution of 0.1-0.2 millimetres, allowing detection of subtle cortical irregularities and assessment of bone density variations that contribute to surface deformation. Three-dimensional reconstruction capabilities enable comprehensive evaluation of complex bone geometry and facilitate accurate measurement of indentation depth, volume, and relationship to surrounding anatomical structures.
CT scanning protocols for tibial indentation evaluation typically employ thin-section acquisition with slice thicknesses of 0.5-1.0 millimetres to optimise spatial resolution whilst minimising radiation exposure. Multiplanar reconstruction techniques allow visualisation of bone architecture in multiple planes, providing comprehensive assessment of cortical integrity and trabecular pattern abnormalities. Quantitative CT analysis can measure bone mineral density with precision comparable to dual-energy X-ray absorptiometry whilst providing superior anatomical detail.
Advanced CT post-processing techniques, including finite element analysis and computational biomechanical modelling, enable prediction of bone mechanical properties and fracture risk assessment. These sophisticated analytical approaches can identify areas of stress concentration that may predispose to progressive deformation or secondary fracture development. Volume rendering techniques facilitate three-dimensional visualisation that enhances understanding of complex bone pathology and assists in surgical planning when intervention is required.
MRI signal characteristics in bone marrow oedema
Magnetic resonance imaging provides superior soft tissue contrast resolution and enables detection of bone marrow abnormalities that may accompany or contribute to tibial indentation formation. Bone marrow oedema, characterised by increased water content within the marrow space, appears as high signal intensity on T2-weighted and STIR sequences whilst demonstrating low signal intensity on T1-weighted images. This imaging pattern often indicates active bone pathology, inflammatory processes, or stress-related changes that may precede structural deformation.
Specialised MRI sequences, including gradient-echo and susceptibility-weighted imaging, can detect microhaemorrhage and trabecular disruption associated with acute bone injury or chronic stress-related changes. Dynamic contrast-enhanced MRI provides information about bone perfusion and may identify areas of compromised vascular supply that could contribute to bone necrosis and subsequent structural collapse. The absence of ionising radiation makes MRI particularly suitable for longitudinal monitoring of bone healing and treatment response assessment.
MRI demonstrates particular utility in differentiating between various pathological processes that may present with similar clinical appearances. Infection, tumour infiltration, and metabolic bone disease each demonstrate characteristic signal patterns that enable accurate diagnosis and appropriate treatment selection. Diffusion-weighted imaging sequences can provide additional diagnostic information by assessing cellular density and tissue perfusion characteristics that further refine diagnostic accuracy.
DEXA scanning for bone density quantification
Dual-energy X-ray absorptiometry scanning represents the gold standard for bone mineral density assessment and plays a crucial role in evaluating metabolic contributions to tibial indentation formation. DEXA technology utilises two different X-ray energy levels to differentiate between bone mineral and soft tissue, providing precise quantification of bone density with excellent reproducibility and minimal radiation exposure. Standard DEXA protocols assess bone density at the proximal femur and lumbar spine, but specialised techniques can evaluate regional bone density in the tibia and other peripheral sites.
Bone density measurements are typically expressed as T-scores and Z-scores that compare individual results to population norms. T-scores compare bone density to healthy young adults of the same gender, whilst Z-scores compare results to age-matched peers. A T-score of -2.5 or lower indicates osteoporosis and significantly increased fracture risk, whilst scores between -1.0 and -2.5 suggest osteopenia or reduced bone density that may predispose to structural abnormalities.
Advanced DEXA analysis techniques, including trabecular bone score assessment and hip structural analysis, provide additional information about bone quality beyond simple density measurements. These sophisticated approaches can identify individuals at increased risk of fracture despite normal bone density measurements, highlighting the importance of bone microarchitecture in determining structural integrity. Serial DEXA measurements enable monitoring of bone density changes over time and assessment of treatment efficacy in patients with metabolic bone disease.
Ultrasound elastography in soft tissue evaluation
Ultrasound elastography represents an emerging imaging technique that provides quantitative assessment of tissue stiffness and elasticity properties surrounding tibial indentations. This non-invasive imaging modality can evaluate soft tissue changes associated with bone pathology, including periosteal thickening, muscle atrophy, or fibrotic tissue formation that may contribute to functional limitations. Real-time elastographic imaging enables dynamic assessment of tissue properties during muscle contraction or passive movement, providing insights into biomechanical alterations associated with bone deformation.
Shear wave elastography techniques measure tissue stiffness by analysing the propagation velocity of acoustic waves through different tissue types. Normal muscle tissue demonstrates specific elasticity values that may be altered in the presence of inflammation, fibrosis, or disuse atrophy associated with chronic bone pathology. The periosteum and surrounding fascial planes can be evaluated for thickening or abnormal stiffness that may indicate active inflammatory processes or chronic tissue changes.
The accessibility and cost-effectiveness of ultrasound elastography make it particularly suitable for serial monitoring of tissue changes during treatment or rehabilitation. This imaging technique can assess treatment response and identify complications such as compartment syndrome or vascular compromise that may develop secondary to tibial pathology. Integration of elastographic findings with other imaging modalities enhances overall diagnostic accuracy and facilitates comprehensive treatment planning for complex cases involving tibial indentation formation.
Advanced imaging protocols should be tailored to individual patient presentations, considering factors such as age, comorbidities, and suspected underlying pathology to optimise diagnostic yield whilst minimising unnecessary radiation exposure and healthcare costs.