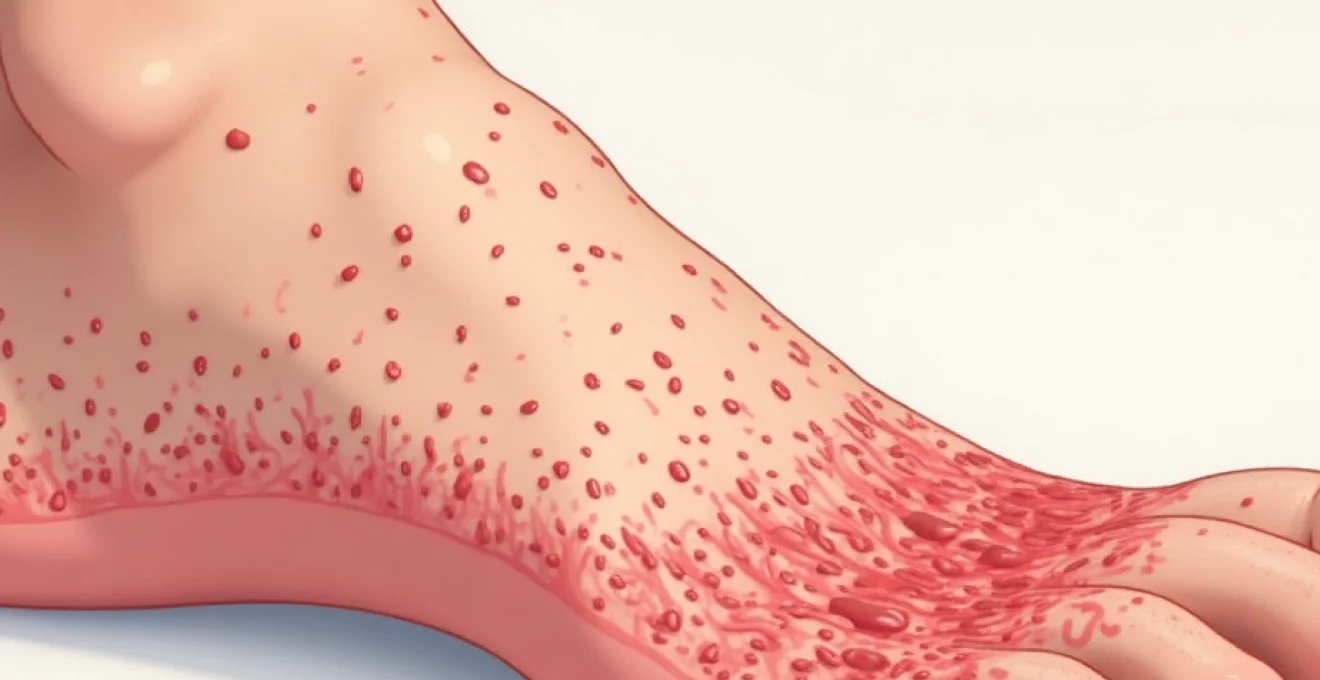
Penile desquamation, or skin peeling on the penis, represents a relatively common dermatological concern that affects men across all age groups. This condition manifests as the shedding of the outer epidermal layer, often accompanied by dryness, irritation, and varying degrees of discomfort. While many instances of penile skin peeling resolve spontaneously or with basic care, understanding the underlying mechanisms and potential causes proves crucial for appropriate management and prevention of complications.
The penile skin possesses unique characteristics that make it particularly susceptible to various dermatological conditions. Its thin, sensitive nature combined with exposure to moisture, friction, and potential allergens creates an environment where desquamation can readily occur. The complexity of penile skin peeling stems from its multifactorial nature , encompassing infectious agents, inflammatory conditions, mechanical trauma, and chemical irritation.
Modern medical understanding recognises that penile desquamation rarely occurs in isolation, typically presenting alongside other symptoms such as erythema, pruritus, burning sensations, or discharge. The clinical presentation often provides valuable diagnostic clues, enabling healthcare professionals to differentiate between various aetiological factors and implement targeted therapeutic interventions.
Common dermatological conditions causing penile desquamation
Dermatological conditions represent the most frequent underlying causes of penile skin peeling, with several distinct pathophysiological mechanisms contributing to epithelial shedding. These conditions often manifest through chronic inflammatory processes that disrupt normal keratinocyte turnover and barrier function. Understanding the specific characteristics of each condition enables more precise diagnosis and treatment selection.
Contact dermatitis from latex condoms and spermicidal lubricants
Contact dermatitis affecting penile tissue commonly results from exposure to latex proteins or chemical irritants found in contraceptive products. Immediate-type hypersensitivity reactions to latex can produce rapid onset of erythema, swelling, and subsequent desquamation within hours of contact. The inflammatory cascade involves IgE-mediated mast cell degranulation, releasing histamine and other mediators that compromise epidermal integrity.
Spermicidal lubricants containing nonoxynol-9 frequently cause irritant contact dermatitis through direct cytotoxic effects on keratinocytes. This mechanism differs from allergic contact dermatitis, as it doesn’t require prior sensitisation and can occur with first exposure. The resulting inflammation disrupts intercellular junctions, leading to accelerated desquamation and barrier dysfunction.
Seborrhoeic dermatitis affecting genital epidermis
Seborrhoeic dermatitis occasionally extends to genital areas, particularly in individuals with widespread disease affecting the scalp, face, and trunk. The condition involves Malassezia species colonisation of sebaceous gland-rich areas, though genital involvement typically occurs through secondary spread rather than primary localisation. The inflammatory response produces characteristic scaling and desquamation patterns.
The pathophysiology involves abnormal sebaceous gland activity combined with altered immune responses to commensal Malassezia yeasts. This creates a chronic inflammatory state characterised by increased keratinocyte proliferation and abnormal differentiation, resulting in visible scaling and peeling. Treatment typically requires antifungal agents combined with anti-inflammatory therapies.
Atopic eczema manifestations on penile shaft tissue
Atopic dermatitis can affect genital skin, producing intense pruritus accompanied by characteristic eczematous changes including desquamation. The condition typically presents in individuals with personal or family histories of atopic diseases such as asthma or allergic rhinitis. Genetic variations in filaggrin expression contribute to impaired barrier function, making affected individuals more susceptible to environmental triggers and secondary infections.
The inflammatory process involves Th2-mediated immune responses producing elevated IgE levels and eosinophilia. Chronic scratching and rubbing exacerbate the condition, creating a cycle of inflammation and desquamation. The intimate location adds psychological distress, often leading to delayed medical consultation and treatment.
Psoriasis plaques and scaling on glans penis
Genital psoriasis affects approximately two-thirds of individuals with generalised psoriasis, though it may occasionally present as the initial manifestation of the disease. Unlike classic psoriatic plaques elsewhere on the body, genital lesions typically appear as smooth, erythematous patches with minimal scaling due to the moist environment. However, desquamation becomes more prominent when the affected area experiences drying or friction.
The underlying pathophysiology involves T-cell mediated inflammation with accelerated keratinocyte turnover, reducing the normal epidermal transit time from 28 days to approximately 3-4 days. This rapid cellular turnover produces the characteristic scaling and silvery appearance, though genital presentations may be more subtle. Treatment requires careful consideration of the sensitive location and potential for systemic absorption.
Infectious aetiologies behind penile epithelial shedding
Infectious causes of penile desquamation encompass bacterial, fungal, and viral pathogens that directly damage epithelial tissue or trigger inflammatory responses leading to skin peeling. These conditions often present with additional symptoms including discharge, odour, and systemic manifestations. Early recognition and appropriate antimicrobial therapy prove crucial for preventing complications and reducing transmission risk.
Candida albicans balanitis and accompanying desquamation
Candidal balanitis represents one of the most common infectious causes of penile desquamation, particularly affecting uncircumcised males due to the warm, moist environment beneath the prepuce. Candida albicans proliferation occurs when normal microbial balance becomes disrupted through antibiotic use, diabetes mellitus, or immunosuppression. The organism produces proteolytic enzymes that directly damage keratinocytes, resulting in characteristic white plaques and subsequent peeling.
The clinical presentation typically includes intense pruritus, erythema, and cottage cheese-like discharge accompanied by progressive desquamation as lesions resolve. Diagnosis relies on microscopic examination of potassium hydroxide preparations or fungal cultures. Topical antifungal agents such as clotrimazole or miconazole provide effective treatment, though systemic therapy may be necessary for recurrent or severe cases.
Bacterial balanoposthitis from staphylococcus aureus
Bacterial infections involving the glans and prepuce can produce significant inflammation leading to secondary desquamation. Staphylococcus aureus commonly colonises compromised penile skin, particularly following minor trauma or in the presence of underlying dermatological conditions. The organism produces various virulence factors including exotoxins that damage epithelial tissues and trigger intense inflammatory responses.
Secondary bacterial infections often complicate primary dermatological conditions, creating a mixed pathological picture requiring comprehensive treatment approaches. Methicillin-resistant Staphylococcus aureus (MRSA) emergence has complicated treatment protocols, necessitating culture and sensitivity testing to guide antibiotic selection. Topical antiseptics and systemic antibiotics may both be required for adequate bacterial eradication.
Herpes simplex virus type 1 and type 2 vesicular eruptions
Herpes simplex virus infections produce characteristic vesicular lesions that rupture to form shallow ulcerations, followed by crusting and eventual desquamation during the healing phase. Both HSV-1 and HSV-2 can affect genital tissues, though HSV-2 demonstrates greater tropism for genital epithelium. The viral replication cycle directly damages infected keratinocytes, causing cell lysis and vesicle formation.
The healing process involves significant inflammatory infiltration and tissue remodelling, often resulting in temporary pigmentary changes and residual scaling. Recurrent episodes typically produce less severe desquamation due to established immune responses, though the location and extent can vary between episodes. Antiviral medications such as aciclovir or valaciclovir reduce symptom duration and viral shedding when initiated early in the disease course.
Human papillomavirus induced hyperkeratosis and peeling
Certain human papillomavirus subtypes produce hyperkeratotic lesions on genital skin that undergo periodic desquamation as part of the viral lifecycle. HPV infection transforms keratinocytes, leading to abnormal proliferation and differentiation patterns that manifest as warts or flat lesions. The viral proteins interfere with normal cell cycle regulation, producing the characteristic hyperkeratotic appearance.
Subclinical HPV infections may present solely as areas of increased desquamation without obvious wart formation. These lesions become more apparent following acetic acid application during clinical examination. Treatment options include topical immunomodulators, cryotherapy, or surgical excision, depending on lesion characteristics and patient preferences.
Mechanical trauma and Friction-Related penile skin damage
Mechanical trauma represents a frequently overlooked cause of penile desquamation, particularly in sexually active individuals or those engaging in frequent masturbation. The penile skin’s delicate nature makes it particularly susceptible to friction-induced damage, especially when insufficient lubrication accompanies sexual activity. Microscopic tears in the epidermis initiate inflammatory cascades that ultimately result in visible peeling and flaking.
Chronic friction produces a cycle of damage and repair that can lead to persistent desquamation patterns. The healing process involves increased keratinocyte proliferation and accelerated differentiation, often producing visible scaling as new cells replace damaged tissue. This mechanism explains why individuals with active sexual lives may experience recurring episodes of penile skin peeling without underlying pathological conditions.
Clothing choices significantly impact friction-related penile skin damage. Tight-fitting underwear made from synthetic materials can create continuous low-level friction throughout daily activities. Cotton-based fabrics allow better air circulation and reduce moisture retention, minimising the risk of friction-induced skin damage. Proper lubrication during sexual activity remains the most effective preventive measure for reducing friction-related desquamation.
The severity of mechanical trauma varies considerably based on individual skin sensitivity, activity intensity, and duration of exposure. Some individuals develop tolerance to friction over time, while others remain consistently susceptible to even minor mechanical irritation. Understanding personal risk factors enables implementation of appropriate preventive strategies to minimise recurring episodes.
Mechanical friction accounts for approximately 30-40% of non-infectious penile desquamation cases, highlighting the importance of addressing lifestyle factors in treatment planning.
Allergic reactions to personal care products and medications
Allergic contact dermatitis affecting penile skin has become increasingly common with the proliferation of personal care products containing complex chemical formulations. The intimate area’s increased permeability and prolonged contact with potentially sensitising substances creates ideal conditions for allergic reaction development. Many individuals remain unaware of the connection between their personal care routines and recurring penile skin problems.
Sodium lauryl sulphate sensitivity in soaps and shower gels
Sodium lauryl sulphate (SLS) represents one of the most common irritant ingredients in personal cleansing products, capable of causing significant penile skin irritation even in individuals without established allergies. SLS disrupts lipid bilayers in the stratum corneum , compromising barrier function and increasing susceptibility to other irritants. The resulting inflammation produces characteristic erythema followed by desquamation as the damaged epithelium regenerates.
The concentration of SLS in products varies considerably, with higher concentrations producing more severe reactions. Even products marketed as “gentle” or “sensitive skin” formulations may contain sufficient SLS to trigger reactions in susceptible individuals. Alternative surfactants such as sodium lauryl glucose carboxylate or coco-glucoside offer gentler cleansing properties with reduced irritation potential.
Topical antibiotic hypersensitivity including neomycin
Topical antibiotics frequently cause allergic contact dermatitis, with neomycin representing one of the most common sensitising agents. The mechanism involves delayed-type hypersensitivity reactions that typically develop 24-72 hours after exposure. Cross-reactivity between related aminoglycoside antibiotics can complicate treatment selection and require careful medication history review.
The clinical presentation often mimics the original condition being treated, leading to continued application and worsening of symptoms. This scenario creates a paradoxical situation where the treatment becomes the primary cause of ongoing inflammation and desquamation. Recognition requires high clinical suspicion and often necessitates patch testing for definitive diagnosis.
Fragrance allergen reactions from aftershaves and deodorants
Fragrance allergies affect approximately 2-3% of the general population, with penile skin being particularly susceptible due to its increased permeability and potential for prolonged contact with scented products. Common sensitising compounds include cinnamaldehyde, eugenol, and various terpenes found in essential oils. The allergic response involves T-cell mediated inflammation that produces characteristic eczematous changes including desquamation.
Many individuals unknowingly expose their genital area to fragrance allergens through transfer from hands after applying scented products or through residual traces in clothing washed with fragranced detergents. Fragrance-free product formulations provide the safest option for sensitive individuals, though distinguishing between “fragrance-free” and “unscented” products requires careful label reading.
Latex protein allergenicity in intimate products
Latex allergy represents a potentially serious medical condition that can produce severe local reactions including extensive desquamation and systemic symptoms. The allergenic proteins in natural rubber latex trigger IgE-mediated responses that can progress from localised skin reactions to anaphylaxis in severely sensitised individuals. Healthcare workers and individuals with frequent latex exposure demonstrate higher sensitisation rates.
Non-latex alternatives including polyurethane and synthetic rubber products provide safe options for latex-allergic individuals. However, some products marketed as “latex-free” may still contain trace amounts of latex proteins sufficient to trigger reactions in highly sensitised individuals. Complete latex avoidance remains the most effective management strategy for confirmed latex allergy.
Hormonal imbalances affecting penile keratinocyte turnover
Hormonal influences on penile skin health remain underappreciated despite significant evidence demonstrating their impact on keratinocyte function and turnover rates. Testosterone and its metabolites directly influence sebaceous gland activity, epidermal thickness, and barrier function through androgen receptor activation. Age-related hormonal changes can alter skin characteristics, making older individuals more susceptible to desquamation and delayed healing responses.
Diabetes mellitus represents a common endocrinological condition significantly impacting penile skin health through multiple mechanisms. Chronic hyperglycaemia alters protein glycation, compromising collagen structure and reducing skin elasticity. Additionally, diabetic individuals demonstrate increased susceptibility to Candida infections and delayed wound healing, both contributing to recurring desquamation episodes. Optimal glycaemic control proves essential for maintaining penile skin health in diabetic patients.
Thyroid dysfunction can produce subtle but significant changes in skin metabolism and turnover rates. Hypothyroidism typically causes dry, scaling skin throughout the body, including genital areas, while hyperthyroidism may increase skin fragility and susceptibility to trauma. Thyroid hormone replacement therapy often improves skin condition in hypothyroid patients, though adjustments may be necessary to achieve optimal results.
Corticosteroid medications, whether applied topically or administered systemically, profoundly impact penile skin structure and function. Long-term corticosteroid use produces skin atrophy, increased fragility, and impaired barrier function, predisposing to recurring desquamation episodes. The anti-inflammatory effects may initially mask symptoms while underlying structural damage progresses, creating a complex clinical picture requiring careful management strategies.
Hormonal factors contribute to approximately 15-20% of chronic penile desquamation cases, emphasising the importance of comprehensive endocrinological assessment in persistent cases.
Clinical assessment and differential diagnosis of penile desquamation
Comprehensive clinical evaluation of penile desquamation requires systematic assessment of presenting symptoms, medical history, and physical examination findings. The diagnostic process must differentiate between infectious and non-infectious causes while identifying potential underlying systemic conditions. Detailed sexual and exposure history
proves crucial for accurate diagnosis and treatment planning. Healthcare professionals must explore potential triggers including new sexual partners, contraceptive methods, personal care products, or medications that may have initiated symptoms.
Physical examination should encompass the entire genital area, including the penile shaft, glans, foreskin, and surrounding skin. Characteristic distribution patterns often provide diagnostic clues, with contact dermatitis typically affecting areas of direct product contact, while infectious causes may demonstrate more widespread involvement. The presence of satellite lesions, lymphadenopathy, or systemic symptoms may indicate specific pathological processes requiring targeted investigation.
Laboratory investigations play a crucial role in confirming suspected diagnoses, particularly for infectious aetiologies. Potassium hydroxide preparations enable rapid identification of fungal elements, while bacterial cultures guide appropriate antibiotic selection. Viral PCR testing provides definitive diagnosis for herpes simplex infections, especially during atypical presentations or initial episodes where serology may remain negative.
Patch testing represents the gold standard for identifying contact allergens in cases of suspected allergic contact dermatitis. This procedure involves applying standardised allergen panels to unaffected skin areas and monitoring for delayed hypersensitivity reactions over 48-96 hours. Common allergens tested include fragrance mixes, preservatives, rubber additives, and topical medications frequently associated with genital sensitivity reactions.
Dermatoscopic examination can reveal subtle morphological features not apparent to naked eye inspection, particularly useful for distinguishing between inflammatory and infectious causes. The presence of specific vascular patterns, scale characteristics, or pigmentary changes may support particular diagnostic hypotheses and guide further investigation strategies.
Accurate diagnosis of penile desquamation requires correlation of clinical presentation with appropriate laboratory investigations, as visual appearance alone may be misleading in up to 40% of cases.
Differential diagnosis must consider the temporal relationship between symptom onset and potential triggering factors. Acute presentations following new exposures suggest allergic or irritant contact dermatitis, while chronic, recurring episodes may indicate underlying dermatological conditions or persistent infectious agents. The response to initial therapeutic interventions often provides additional diagnostic information, with rapid improvement suggesting correct identification of the underlying cause.
Biopsy procedures may be necessary in atypical cases or when malignancy cannot be excluded based on clinical presentation alone. Histopathological examination can differentiate between various inflammatory patterns and identify specific infectious agents or dysplastic changes requiring specialised treatment approaches. Immunofluorescence studies may be indicated when autoimmune blistering diseases are suspected, though these conditions rarely present with isolated genital involvement.
The diagnostic process must also consider psychological factors that may influence symptom reporting and treatment compliance. Anxiety and embarrassment associated with genital symptoms can lead to delayed presentation, self-medication attempts, or incomplete history disclosure. Healthcare providers must create supportive environments encouraging honest communication about symptoms, sexual practices, and treatment preferences to ensure optimal diagnostic accuracy and therapeutic outcomes.